We explain what water is and what its composition is. What is it for and what is its importance. Also, what is drinking water.

What is water
Water is a substance liquid devoid of odor, taste and color, which exists in a more or less pure state in the nature and covers a significant percentage (71%) of the surface of the planet Earth. Furthermore, it is a fairly common substance in the Solar system and the universe, although in the form of vapor (its gaseous form) or ice (its solid form).
On our planet, water is contained mainly in the seas Y oceans (96.5%), in glaciers and polar caps (1.74%) and in aquifer and permafrost deposits (1.72%). The rest of the planet's water (0.04%) is distributed among lakes, humidity of the soils, atmospheric vapor, reservoirs, rivers and in the body of the living beings.
Water is essential for life as we know it, and the world's first life forms took place inside it. It has also occupied a central place in the imagination of human civilizations, where it has generally been attributed to some deity, or to the mythical flood with which the gods devastate the gods. cultures wayward.
On the other hand, the planet's water is subjected to a natural cycle known as the water cycle or hydrological, in which liquid waters evaporate due to the action of Sun and amount to atmosphere in gaseous form, then they condense into clouds and fall back to the ground as rain. This circuit is vital for the climatic and biological stability of the planet.
Water composition

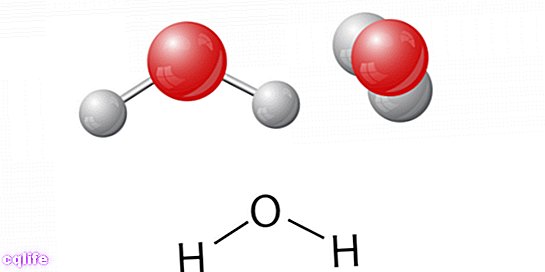
A molecule of water contains only two elements: an oxygen atom and two hydrogen (H2O) linked covalently. This was discovered in 1782 thanks to the chemist Henry Cavendish, since water was thought of as an element since ancient times.
Water has a non-linear structure. Its two hydrogen atoms are bonded to the oxygen atom and form an angle of 104.5º to each other. This distribution of its atoms, added to the high electronegativity value of the oxygen atom, generates the formation of an electric dipole that determines the polarity of the water.
Water is considered the universal solvent, since most substances can be dissolved in it. These substances are polar and called hydrophilic. On the other hand, nonpolar (apolar) substances, such as oil or gasoline, are called hydrophobic and do not dissolve in water.
Water properties
- It is colorless, odorless and tasteless.
- Has good electric conductivity when it has ions dissolved in it and it is practically like an electrical insulator in its pure state.
- In its pure state it is diamagnetic, that is, it repels magnetic fields.
- It has a high surface tension (energy required to increase its surface area) and, therefore, has a high resistance to increasing its surface.
- It is extremely adhesive. Adhesion is the attraction of molecules of one type by molecules of the same or another type.
- It has a very stable density but when the temperature drops, unlike other liquids, its density decreases when it passes to solid state. That is why we see that ice floats in liquid water.
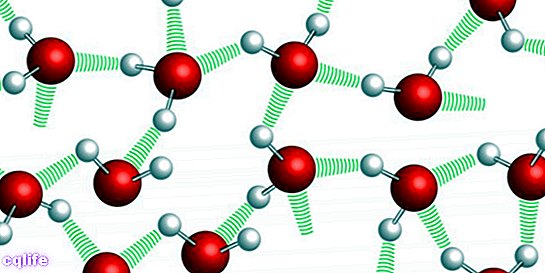
- It has a high melting point and of boiling (100 ⁰C) because water molecules have hydrogen bonding interactions with each other, which prevents water from passing into the liquid state or gaseous, as the case may be, at a lower temperature.
Function of water
Water fulfills vital functions on the planet, whether in the aquatic ecosystems or terrestrial. It is a vital means of transporting nutrients and is essential for the photosynthesis of the plants.
In the human body, water is the protagonist of a large number of processes:
- It constitutes the vital medium for most of the cells of the body.
- It carries dissolved substances and makes up a huge percentage of blood.
- It facilitates the excretion of substances forming part of the urine, feces, sweat and other excretions.
- Maintains the temperature homogeneous body, regulating body temperature.
- Provides electrolytes and minerals essential for the electrical functioning of the body.
On the other hand, the great masses of water on the planet are a means of human distraction and recreation, for example, beaches, water sports etc. It is also one of the fundamental inputs in industries and the main input for the hygiene everyday.
Importance of water

The massive presence of liquid water on the planet is one of its main differences with respect to neighboring planets, and it is what allowed the birth and flourishing of life. Let us remember that the first steps of evolution they occurred at the microscopic level in the seas.
On the other hand, water, ice, steam and its hydrological cycle maintain climatic and atmospheric stability by allowing the cooling of the planet, which receives daily the sunlight. It also hydrates soils, making them fertile for plant life and for agricultural activity, and keeps waste substances circulating so that they are distributed in less harmful quantities in the environment.
Drinking water
Drinking water is all water that is suitable for consumption human, both to drink and to prepare food or meals. There are maximum values of pH, minerals, salts and microorganisms that distinguish drinking water from water not suitable for consumption. This means that drinking water is low compared to large bodies of non-potable water, such as the sea or the rain.
Fortunately, there are water purification initiatives that combat the constant flow of toxic substances and pollutants that human beings throw into large bodies of water, a product of industry or life urban. Desalination plants, ozonation, irradiation and other mechanisms of purification are responsible for this function.
