We explain what metals are, how they are classified and what their physical properties are. Examples of metals and what are non-metals.

What are metals?
In the field of chemistry, are known as metals or metallic those elements of the Periodic table that are characterized by being good drivers of the electricity and from heat. These elements have high densities and are generally solid at room temperature (except for mercury). Many, furthermore, may reflect the light, which gives them their characteristic shine.
Metals are the most numerous elements in the Periodic Table and some are among the most abundant in the Earth crust. A part of them is usually found in a state of greater or lesser purity in the nature, although most are part of minerals from the earth's subsoil and must be separated by the human being to use them.
Metals have characteristic bonds called "metal links”. In this type of bond, the metal atoms are linked together in such a way that their atomic nuclei join with the valence electrons (electrons located in the last electronic shell, that is, the outermost electrons), which form a kind of "cloud" around it. Thus, in the metallic bond, the metallic atoms are located very close to each other, and all are "immersed" in their valence electrons, forming the metallic structure.
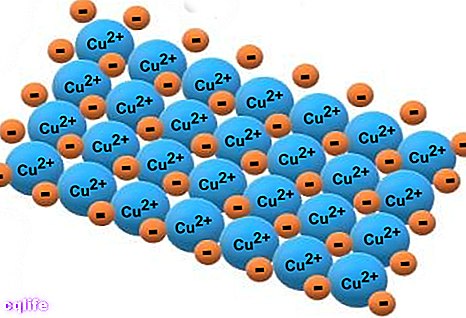
On the other hand, metals can form ionic bonds with non-metals (for example, chlorine and fluorine), which results in the formation of salts. This type of bond is formed by the electrostatic attraction between ions of different sign, where metals form positive ions (cations) and non-metals form negative ions (anions). When these salts dissolve in water, they dissociate into their ions.
Even the alloys of one metal with another (or with a nonmetal) continue to be metallic materials, as is the case with steel and bronze, although they are mixtures homogeneous.
Metals have served the humanity since time immemorial thanks to its ideal character to form tools, statues or structures of all kinds, due to its particular physical properties:
- Malleability. When subjected to compression, some metals can form thin sheets of homogeneous material.
- Ductility. When subjected to tensile forces, some metals can form wires or strands of homogeneous material.
- Tenacity. Ability to resist fracture, when subjected to forces abrupt (bumps, falls, etc.).
- Mechanical strength. Ability to withstand traction, compression, torsion, and other forces without yielding in its structure physical or deformed.
In addition, their shine makes them ideal for forging jewelry and decorative elements and their good conduction of the electricity makes them indispensable in the transmission of the electric current in modern systems of electric power.
Metal types
Metallic elements can be of various types, according to which they are grouped in the Periodic Table. Each group has shared properties:
- Alkali metals. They are shiny, soft and very reactive under normal conditions of Pressure Y temperature (1 atm and 25º C), so they are never pure in the nature. They have low densities and are good conductors of heat and electricity. They also have low melting and boiling points. In the Periodic Table they occupy group I. In this group is also hydrogen (which is not a metal).
- Alkaline earth metals. They are located in group II of the Periodic Table. Its name comes from the alkaline properties of its oxides (formerly called "earths"). They are usually harder and less reactive than alkaline ones. They are bright and good conductors of heat and electricity. They have low density Y color.
- Transition metals. Most metals belong to that category. They occupy the central region of the Periodic Table and almost all are hard, with high melting points Y boiling, and good conduction of heat and electricity.
- Lanthanides. Also called lanthanoids, they are the so-called "rare earths" of the Periodic Table, which with actinides form the "internal transition elements". They are very similar elements to each other, and despite their name, they are very abundant on the earth's surface. They have magnetic behaviors (when they interact with a magnetic field, for example, the magnetic field that generates a magnet) and spectral (when radiation falls on them) very characteristic.
- Actinides. Together with rare earths, they form the "internal transition elements", and are very similar to each other. They present high atomic numbers and many of them are radioactive in all their isotopes, which makes them extremely rare in nature.
- Transactinides. Also called “super heavy elements”, they are those that exceed in atomic number the heaviest of the actinides, lawrencio. All the isotopes of these elements have a very short half-life, are all radioactive and have been obtained by synthesis in a laboratory, so they have the names of the physicists responsible for their creation.
Examples of metals

- Alkaline Lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), francium (Fr).
- Alkaline earths. Beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra).
- Transition metals. Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), nickel (Neither), copper (Cu), zinc (Zn), yttrium (Y), zirconium (Zr), niobium (Nb), molybdenum (Mo), technetium (Tc), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), cadmium (Cd), lutetium (Lu), hafnium (Hf), tantalum (Ta), tungsten (W), rhenium (Re), osmium (Os), iridium (Ir), platinum (Pd), gold (Au), mercury (Hg), lawrence (Lr), rutherfordium (Rf), dubnium (Db), seaborgium (Sg), bohrio (Bh), hasium (Hs), meitnerium (Mt), darmstadium (Ds), roentgenium (Rg), copernicium (Cn).
- Rare earths. Lanthanum (La), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb), Lutetium (Lu).
- Actinides. Actinium (Ac), thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), americium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No), lawrencio (Lr).
- Transactinides. Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrio (Bh), Hassium (Hs), Meitnerium (Mt), Darmstadium (Ds), Roentgenium (Rg), Copernicium (Cn), Nihonium (Nh), flerovio (Fl), moscovio (Mc), livermorio (Lv), teneso (Ts).
What are non-metals?

Nonmetals are elements with very different properties from those of metals, although there are also compounds called metalloids, which have properties and characteristics intermediate between metals and non-metals. Nonmetals form covalent bonds when they form molecules among them. These compounds, unlike metals, are not good conductors of electrical current and heat, nor are they shiny.
Oxygen, carbon, hydrogen, nitrogen, phosphorus and sulfur, which are the fundamental elements for the life, are part of the non-metals. These non-metallic elements can be solid, liquid or gaseous.
They are mainly classified as:
- Halogens Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astate (At) and tenese (Ts).
- Noble gases. Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), oganeson (Og).
- Other non-metals. Hydrogen (H), carbon (C), sulfur (S), selenium (Se), nitrogen (N), oxygen (O) and phosphorus (P).
