We explain what matter and energy are, the characteristics of each one and how they were studied. Also, the relationship between the two.

What are matter and energy?
Our universe integer is composed of matter Y Energy, in its many forms, presentations and capacities. In fact, the two basic disciplines with which we try to understand the fundamental laws that govern it, the physical and the chemistry, deal with the relationships between these two elements: the substance that composes things and their ability to transfer heat or make a job.
From an intuitive point of view, we understand matter as that which we can touch, which is concrete and occupies a place in the universe. On the other hand, the energy cannot be touched, which is only perceived in its different manifestations: heat, light, movement, etc. The things around us have at the same time a mass own and a variable amount of energy, depending largely on the state in which they are.
These are two fundamental concepts, closely related to each other, between which there are certain equivalences.For example, it is possible to transform mass into energy, something that happens daily inside the stars, through intense nuclear reactions, or within our own organisms, when we decompose the food that we ingest and extract from them chemical energy To keep us alive
Matter
Matter is what makes up living things, objects, air, and more.Matter is defined as that which extends in a certain region of space-time, that has a certain amount of energy and that is subject to changes in the time. Its name comes from the Latin mater, "Mother", since it is about the substance matrix of things, that is, of what originates or composes them.
In general, physics attributes three fundamental features or properties to matter:
- It has a specific mass, which is evidenced in a weight, a volume and quantifiable dimensions.
- It occupies a place in the space, which cannot be occupied at the same time by another body.
- Lingers in the weatherAlthough not necessarily in the same way: ice is certainly matter, and it does not stop being so when it melts or when the water that makes it up evaporates. These changes in your physical condition (or state of matter aggregation) depend on the amount of energy you have.
The study of matter dates back to classical antiquity, and occupied many thinkers and philosophers throughout history. In fact, it was the ancient Greeks who first formulated the atomistic theory, that is, those who thought that matter could be composed of tiny and indivisible particles of different types.
This idea was rescued much later, in the boom rationalist of the seventeenth century, and was fundamental in the field of study of chemistry, heir in turn to the alchemy medieval.
According to current physics study models, only about 5% of the appreciable universe is made up of ordinary matter, while the so-called "dark matter”Whose operation is still unknown, occupies 23%. The latter is supposed to be a non-mass form of matter, that is, devoid of mass, whose presence can only be intuited by the way in which they affect stars and the energy around you.
Energy
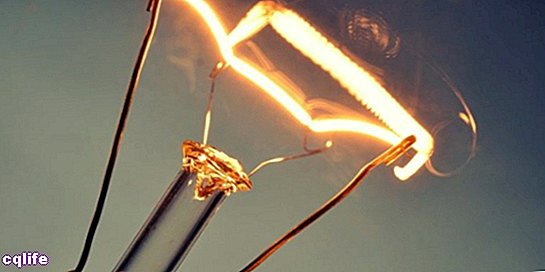
In physics, energy is defined as the ability to do work, that is, to act, arise or set in motion. Absolutely all bodies They possess a certain amount of energy, related to their state of rest, movement or vibration, for example, but which manifests itself in very different ways.
Thus, it is possible to speak of many types of energy: caloric energy, chemical energy, Kinetic energy, electric power, potential energy, internal energy, etc.
The word energy comes from the Greek energetic, "Activity", a term that first appeared in the writings of Aristotle (384-322 BC) in the fourth century BC. C., and retaken by the modern naturalists and of the late Middle Ages.
It has been given many other names throughout history, such as "living force" (vis viva), "Power" or even "spirit", depending on the context. This is largely due to the fact that the study of the different types of energy had their origin separately, as more and more forms of energy present in the universe were discovered.
Energy can be perceived, generally, in its various manifestations, since in the abstract it is not something perceptible. Instead, heat, light, movement, or activity can be perceived with the naked eye, and their effects on matter can be studied without difficulty. Thus, energy would become a physical quantity, which we can measure in its different appearances.
We must also consider that the amount of energy in the system it tends to be constant, so that it cannot be created or destroyed, only transformed. In fact, it is in continuous transformation all the time: the chemical energy stored in food is converted into mechanical energy when we move, or into electrical energy in our nervous system.
Likewise, the electrical energy from the plug is converted into light energy when we turn on the lamp, or into heat energy thanks to the water heater.
Matter and energy
The relationships between matter and energy have been the object of study of physicists for centuries. We know that a change in the energy levels of matter affects its shape and its state of aggregation, which we have seen since we learned to melt metals.
Later, the knowledge of chemistry gave us a much greater understanding of how to transform matter: no longer change the configuration of its particles, but break the links between the particles. atoms and get different substances.
In fact, humanity's greatest achievement in that regard has been the discovery of the atomic Energy and its manipulation for peaceful purposes, that is, in the construction of power plants in which heavy atoms fuse to generate large amounts of heat energy.
All of this was possible thanks to the theories of Albert Einstein (1879-1955) and other important physicists, and especially their formula for the equivalence between mass and energy (E = mc2), known as the Theory of relativity.
