- What is pH?
- What is the relationship between acidity level and pH?
- The pH measurement scale
- Examples of acidic, basic and neutral compounds
- How is pH measured?
- What are Buffer solutions or Buffers?
We explain what pH is and what instruments are used to measure it. The pH scale and examples of acidic, neutral and base compounds.
The pH is nothing more than the indicator of the potential of hydrogens.What is pH?
The pH is a measurement that serves to establish the level of acidity or alkalinity of a solution. The "p" is for "potential", that's why the pH is called: hydrogen potential.
It is expressed as the negative base 10 logarithm of the concentration of ions hydrogen. The following equation represents this definition:
Equation 1: Equations to calculate pH and POH.
On the other hand, pOH is a measure of the concentration of hydroxyl ions in a dissolution. It is expressed as the negative base 10 logarithm of the hydroxyl ion concentration and, unlike pH, is used to measure the alkalinity level of a solution.
An additional piece of information is that in aqueous solution at 25 ºC, the sum of pH and pOH is equal to 14.
What is the relationship between acidity level and pH?
Acidic solutions have a high amount of hydrogen ions. This means that they have low pH values (see equation 1) and, therefore, their acidity level is high. Thus, a solution will be more acidic or less acidic depending on the amount of hydrogen ions it has.
On the other hand, basic (alkaline) solutions have low amounts of hydrogen ions. This means that they have high pH values (see equation 1) and, therefore, their acidity level is low.
The pH measurement scale
The pH scale is used to measure the degree of acidity of a solution and, since pH is related to pOH (see equation 1), then knowing the degree of acidity of a solution, we can also know its degree of basicity.
Thus, the pH scale goes from 0 to 14. For example, substances with a pH value = 0 are the most acidic (least basic), those with pH = 7 are neutral, and those with pH = 14, are the least acidic (most basic).
Examples of acidic, basic and neutral compounds
Examples of acidic compounds
- Acids batteries. They have pH values between 0 and 1. Their acid level is so strong that it is harmful to the species.
- Acid rain. It is a phenomenon that occurs due to the accumulation of acids from fossils and fuels. These rains can take pH values between 2 and 5 on the pH scale. When the pH approaches 2 it can produce the death Of fishes, plants and others species. When the pH approaches 5 it produces less damage, but it still affects aquatic and terrestrial life.
- Lemon juice. It has pH values between 2 and 3.
- Coffee. It has a pH value = 5, or very close values.
Examples of neutral compounds
- Blood
- Milk
Examples of basic compounds
- Milk of magnesia. In the pH table it is located between the values 10 and 11. This product is of consumption
- Bleach or chlorine. It has pH values around 13. It is used for cleaning the home, bathrooms, kitchen and has the power to discolor clothes.
How is pH measured?
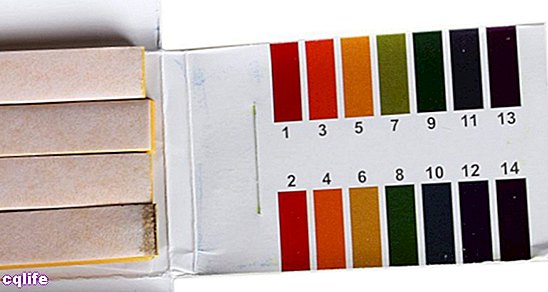
Litmus paper allows us to measure pH.
The way to distinguish between an acidic compound and a basic one is by measuring its pH value. Today there are numerous methods to measure the pH of a substance.
- Using acid-base indicators. Indicators are compounds that change from color by changing the pH of the solution in which they are found. For example, phenolphthalein is a liquid that turns pink if added to a base and turns colorless if added to an acid. Another example is litmus paper: if a fragment is immersed in an acid solution it turns red-orange, and if it is immersed in a basic solution it darkens, taking on a blue color. There are also some types of litmus paper with more specific color scales that indicate more accurate pH values.
- Using a potentiometer or pH meter. It is an electronic equipment that directly gives us the pH value of a solution. Measuring pH using this equipment is more accurate than using litmus paper.
What are Buffer solutions or Buffers?
Buffer solutions or Buffers are solutions that have the property of keeping the pH of a solution constant, even when certain amounts of acid or base.
Buffer solutions are of vital importance in regulating the pH of many biological processes, because for many of them to occur it is necessary that the pH of the medium in which they occur remains constant.
