- What is a polymer?
- Natural polymers
- Synthetic polymers
- Properties and characteristics of polymers
- Examples of polymers
We explain what polymers are, their classification, properties and characteristics. Also, natural and synthetic polymers.
What is a polymer?
In chemistry, polymers are a type of macromolecules made up of chains of simpler units, called monomers, linked together by means of covalent bonds. Its name comes from the Greek polys ("Many") and mere ("segment").
They are generally molecules Organic substances of enormous importance in both the natural and industrial world. These molecules include DNA in ours cells, the starch of plants, the nylon and most of the plastics.
At the end of the 19th century and the beginning of the 20th, it was discovered how to manipulate them. Thus the handling of materials by the company was forever revolutionized. humanity.
- If classified according to their origin, polymers can be:
- Natural polymers. Its origin is biological.
- Synthetic polymers. They are created entirely by the human being.
- Semi-synthetic polymers. They are created by transformation of natural polymers.
- If they are classified according to their composition, we can distinguish between:
- Organic polymers. They have a main chain of atoms carbon.
- Organic vinyl polymers. Similar to organics, but with carbon-carbon double bonds. They include polyolefins, styrenics, halogenated vinyl, and acrylic.
- Non-vinyl organic polymers. They have oxygen and / or nitrogen atoms in their main chain, in addition to carbons. They include polyesters, polyamides, and polyurethanes.
- Inorganic polymers. Based on other elements such as sulfur (polysulfides) or silicon (silicone).
- If they are classified according to their reaction to increasing the temperature, we can distinguish between:
- Elastomeric polymers. They deform with increasing temperature, but regain their original shape.
- Thermoset polymers. When they rise, their temperature breaks down chemically. They do not deform, that is, the material does not flow.
- Thermoplastic polymers. As the temperature rises, they melt and go to the liquid state, but when they cool down, they go back to solid state.
Natural polymers
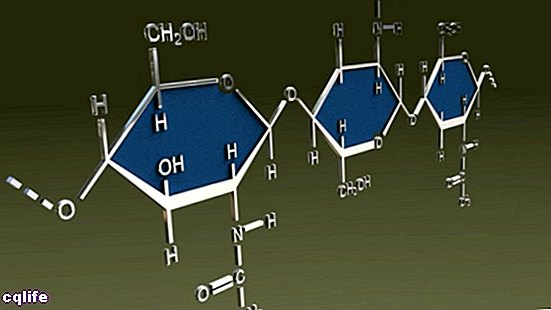
Natural polymers exist as such in the nature, What biomolecules Y compounds that make up the body of living creatures. The appearance of natural polymers in the world represented an important point in the complexity biochemistry of the life.
These include the vast majority of protein, nucleic acids, polysaccharides (complex sugars, such as plant cellulose and chitin from mushrooms), rubber or vegetable rubber.
Synthetic polymers

The first synthetic polymer was created in 1907: Bakelite, a durable and low-cost material. cost. Its great industrial success was largely due to its simple and inexpensive manufacture, using phenol and formaldehyde. Much progress has been made since then in obtaining new and more powerful materials of organic origin, particularly in the petrochemical industry.
Polymers can be created in the laboratory by the union of specific monomers in a chain, using organic or inorganic inputs, under controlled temperature conditions, Pressure and presence of catalysts. Thus, a chain or step reaction is generated that results in the generation of the compound.
Properties and characteristics of polymers
Generally speaking, polymers are bad electric conductors, which is why they are often used as insulators in the electrical industry, for example, plastic as cable wrapping. However, there are conductive polymers, created in 1974, whose applications are still being studied today.
Temperature, on the other hand, is an important factor in the behavior of polymers. At low temperatures they become hard, brittle, similar to glass, while at normal temperatures they tend to elasticity. If the temperature rises towards your melting point, some begin to lose their shape and others can decompose.
Examples of polymers

Some of the best known and most important human polymers are:
- Polyvinylchloride. Also known as PVC and with the general formula (C2H3Cl) n, it is obtained from the polymerization of vinyl chloride units. It is the derivative of the most versatile plastic known and is used for all types of packaging, footwear, coatings, hoses and even pipes.
- Polystyrene. Known as PS, it is obtained from styrene monomers, and can obtain very diverse results: more or less transparent, more or less brittle, or even very dense and waterproof variants. It was synthesized for the first time in Germany in 1930 and since then about 10.6 million tons have been produced annually in the world.
- Polymethylmethacrylate. Abbreviated with the acronym PMMA, it is a typical engineering plastic, and it is one of the most competitive in terms of its industrial applications, since it is extremely transparent and resistant.
- Polypropylene. Referred to in abbreviations as PP, it is a thermoplastic polymer, partially crystalline and made from propylene or propene. It is used in packaging of food, tissues, laboratory equipment and transparent films or films to cover objects.
- Polyurethane. These polymers are obtained by combining hydroxyl bases and diisocyanates, and can be thermoplastic or thermosetting. They are frequently used in the footwear industry, painting, synthetic textile fibers, packaging, condoms or components of machines and vehicles.
