- What are the laws of thermodynamics?
- Origin of the laws of thermodynamics
- First Law of Thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
- Zero law of thermodynamics
We explain what the laws of thermodynamics are, what is the origin of these principles and the main characteristics of each one.

What are the laws of thermodynamics?
The laws of thermodynamics (or the principles of thermodynamics) describe the behavior of three fundamental physical quantities, the temperature, the Energy and theentropy, which characterize thermodynamic systems. The term "thermodynamics" comes from the Greek thermos, What does it mean "heat", Y dynamos, What does it mean "force”.
Mathematically, these principles are described by a set of equations that explain the behavior of thermodynamic systems, defined as any object of study (from a molecule or a human being, until atmosphere or boiling water in a saucepan).
There are four laws of thermodynamics and they are crucial to understanding the physical laws of universe and the impossibility of certain phenomena such as the movement perpetual.
Origin of the laws of thermodynamics
The four principles of thermodynamics They have different origins, and some were formulated from the previous ones. The first to be established, in fact, was the second, the work of the French physicist and engineer Nicolás Léonard Sadi Carnot in 1824.
However, in 1860 this principle was formulated again by Rudolf Clausius and William Thompson, then adding what we now call the First Law of Thermodynamics. Later the third appeared, also known as the "Nerst postulate" because it arose thanks to the studies of Walther Nernst between 1906 and 1912.
Finally, the so-called "zero law" appeared in 1930, proposed by Guggenheim and Fowler. It should be said that not in all areas it is recognized as a true law.
First Law of Thermodynamics

The first law is called the "Law of Conservation of Energy" because it dictates that in any system isolated from its environment, the total amount of energy will always be the same, even though it can be transformed from one form of energy to different ones. Or in other words: energy cannot be created or destroyed, only transformed.
Thus, by supplying a given quantity of heat (Q) to a physical system, its total quantity of energy can be calculated as the heat supplied minus thejob (W) performed by the system on its surroundings. Expressed in a formula: ΔU = Q - W.
As an example of this law, let's imagine an airplane engine. It is a thermodynamic system that consists of fuel that chemically reacts during the process of combustion, releases heat and does work (that makes the plane move). So: if we could measure the amount of work done and heat released, we could calculate the total energy of the system and conclude that the energy in the engine remained constant during the flight: energy was neither created nor destroyed, rather it was made change of chemical energy to caloric energy YKinetic energy (movement, that is, work).
Second law of thermodynamics
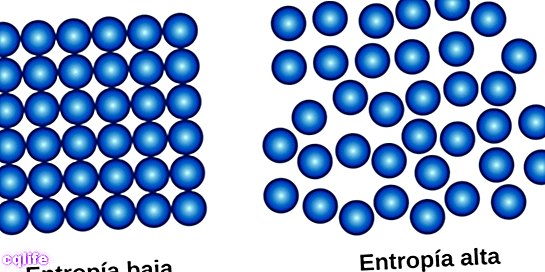
The second law, also called the «Law of Entropy», can be summarized in that the quantity of entropy in the universe tends to increase in the weather. This means that the degree of disorder of the systems increases until reaching a point of equilibrium, which is the state of greatest disorder of the system.
This law introduces a fundamental concept in physics: the concept of entropy (represented by the letter S), which in the case of physical systems represents the degree of disorder. It turns out that in every physical process in which there is a transformation of energy, a certain amount of energy is not usable, that is, it cannot do work. If you can't do work, in most cases that energy is heat. That heat that the system releases, what it does is increase the disorder of the system, its entropy. Entropy is a measure of the disorder of a system.
The formulation of this law establishes that the change in entropy (dS) will always be equal to or greater than theheat transfer (dQ), divided by the temperature (T) of the system. That is, that: dS ≥ dQ / T.
To understand this with an example, it is enough to burn a certain amount of matter and then collect the resulting ashes. When weighed them, we will verify that it is less matter than what was in its initial state: part of the matter was converted into heat in the form of gases that they cannot do work on the system and that they contribute to its disorder.
Third law of thermodynamics

The third law states that the entropy of a system that is brought to absolute zero will be a definite constant. In other words:
- Upon reaching absolute zero (zero in Kelvin units), the processes of physical systems stop.
- Upon reaching absolute zero (zero in Kelvin units), the entropy has a constant minimum value.
It is difficult to reach the so-called absolute zero (-273.15 ° C) on a daily basis, but we can think about this law by analyzing what happens in a freezer: food that we deposit there will get so cold that the biochemical processes inside it will slow down or even stop. That is why its decomposition is delayed and its consumption for much longer.
Zero law of thermodynamics
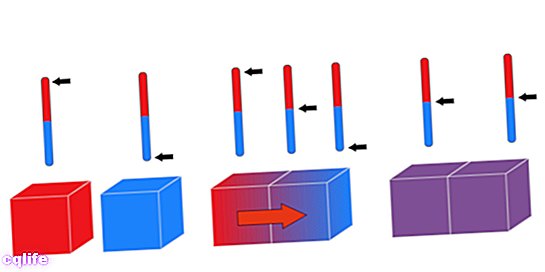
The "zero law" is known by that name although it was the last to run. Also known as Law of Thermal Equilibrium, this principle dictates that: “If two systems are in thermal equilibrium independently with a third system, they must also be in thermal equilibrium with each other ”. It can be logically expressed as follows: if A = C and B = C, then A = B.
This law allows us to compare the thermal energy of three different bodies A, B, and C. If body A is in thermal equilibrium with body C (they have the same temperature) and B also has the same temperature as C, then A and B have the same temperature.
Another way of stating this principle is to argue that when two bodies with different temperatures come into contact, they exchange heat until their temperatures equalize.
Everyday examples of this law are easy to find. When we get into cold or hot water, we will notice the difference in temperature only during the first minutes since our body will then enter into thermal equilibrium with theWater and we will no longer notice the difference. The same happens when we enter a hot or cold room: we will notice the temperature at first, but then we will stop perceiving the difference because we will enter into thermal equilibrium with it.
