- What is a chemical reaction?
- Physical and chemical changes in matter
- Characteristics of a chemical reaction
- How is a chemical reaction represented?
- Types and examples of chemical reactions
- Importance of chemical reactions
- Speed of a chemical reaction
We explain what a chemical reaction is, the types that exist, their speed and other characteristics. Also, physical and chemical changes.
What is a chemical reaction?
Chemical reactions (also called chemical changes or chemical phenomena) are thermodynamic processes of transformation of the matter. Two or more are involved in these reactions substances (reagents or reactants), which change significantly in the process, and can consume or release Energy to generate two or more substances called products.
Every chemical reaction subjects matter to a chemical transformation, altering its structure and molecular composition (unlike Physical changes that only affect its shape or State of aggregation). Chemical changes generally produce new substances, different from what we had in the beginning.
Chemical reactions can occur spontaneously in nature (without human intervention), or they can also be generated by humans in a laboratory under controlled conditions.
Many of the materials that we use on a daily basis are obtained industrially from simpler substances combined through one or more chemical reactions.
Physical and chemical changes in matter
Physical changes in matter are those that alter its shape without changing its composition, that is, without modifying the type of substance in question.
These changes have to do with changes in the aggregation state of matter (solid, liquid, gaseous) and other physical properties (color, density, magnetism, etc).
Physical changes are usually reversible since they alter the shape or state of matter, but not its composition. For example, when boiling Water We can turn a liquid into a gas, but the resulting vapor is still made up of water molecules. If we freeze the water, it goes to the solid state but it is still chemically the same substance.
Chemical changes alter the distribution and bonding of the atoms of matter, achieving that they are combined in a different way, thus obtaining substances different from the initial ones, although always in the same proportionSince matter cannot be created or destroyed, only transformed.
For example, if we react water (H2O) and potassium (K), we will obtain two new substances: potassium hydroxide (KOH) and hydrogen (H2). This is a reaction that normally releases a lot of energy and is therefore very dangerous.
Characteristics of a chemical reaction
Chemical reactions are generally irreversible processes, that is, they involve the formation or destruction of chemical links between the molecules of the reactants, generating a loss or gain of energy.
In a chemical reaction, matter is deeply transformed, although sometimes this recomposition cannot be seen with the naked eye. Still, the proportions of the reactants can be measured, which is dealt with by stoichiometry.
On the other hand, chemical reactions generate certain products depending on the nature of the reactants, but also on the conditions in which the reaction occurs.
Another important issue in chemical reactions is the speed at which they occur, since the control of their speed is essential for their use in the industry, medicine etc. In this sense, there are methods to increase or decrease the speed of a chemical reaction.
An example is the use of catalysts, substances that increase the speed of chemical reactions. These substances do not take part in the reactions, they only control the rate at which they occur. There are also substances called inhibitors, which are used in the same way but cause the opposite effect, that is, they slow down reactions.
How is a chemical reaction represented?
Chemical reactions are represented by chemical equations, that is, formulas in which the participating reagents and the products obtained are described, often indicating certain conditions inherent to the reaction, such as the presence of heat, catalysts, light, etc.
The first chemical equation in history was drawn up in 1615 by Jean Begin, in one of the first treatises on chemistry, the Tyrocinium Chymicum. Today they are of common teaching and thanks to them we can more easily visualize what is happening in a certain reaction.
The general way to represent a chemical equation is:
Where:
- A and B are the reactants.
- C and D are the products.
- to, b, c Y d are the stoichiometric coefficients (they are numbers that indicate the amount of reactants and products) that must be adjusted so that there is the same amount of each element in the reactants and in the products. In this way, the Law of Conservation of Mass is fulfilled (which establishes that the mass it is neither created nor destroyed, it only transforms).
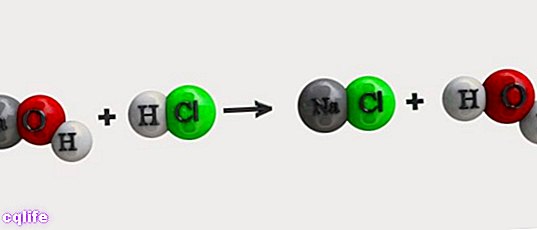
Types and examples of chemical reactions
Chemical reactions can be classified according to the type of reactants that react. Based on this, inorganic chemical reactions and organic chemical reactions can be distinguished. But first, it is important to know some of the symbols that are used to represent these reactions through chemical equations:
Inorganic reactions. Involve inorganic compounds, and can be classified as follows:
- According to the type of transformation.
- Synthesis or addition reactions. Two substances combine to result in a different substance. For example:
- Decomposition reactions. A substance breaks down into its simple components, or one substance reacts with another and breaks down into other substances that contain its components. For example:
- Displacement or substitution reactions. A compound or element takes the place of another in a compound, replacing it and leaving it free. For example:
- Double substitution reactions. Two reactants exchange compounds or chemical elements simultaneously. For example:
- Synthesis or addition reactions. Two substances combine to result in a different substance. For example:
- According to the type and form of the energy exchanged.
- Endothermic reactions. Heat is absorbed so that the reaction can occur. For example:
- Exothermic reactions. Heat is given off when the reaction occurs. For example:
- Endoluminous reactions. Needed light for the reaction to occur. For example: photosynthesis.
- Exoluminous reactions. Light is given off when the reaction occurs. For example:
- Endoelectric reactions. Needed electric power for the reaction to occur. For example:
- Exoelectric reactions. Electrical energy is released or generated when the reaction occurs. For example:
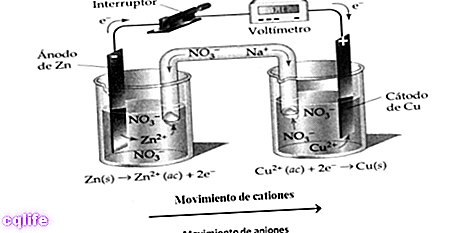
- Endothermic reactions. Heat is absorbed so that the reaction can occur. For example:
- According to the reaction speed.
- Slow reactions The amount of reagents consumed and the amount of products formed in a given time is very little. For example: the oxidation of iron. It is a slow reaction, which we see on a daily basis in iron objects that are rusty. If this reaction were not slow, we would not have very old iron structures in today's world.
- Quick reactions. The amount of reagents consumed and the amount of products formed in a given time is great. For example: the reaction of sodium with water is a reaction that, in addition to occurring rapidly, is very dangerous.
- Slow reactions The amount of reagents consumed and the amount of products formed in a given time is very little. For example: the oxidation of iron. It is a slow reaction, which we see on a daily basis in iron objects that are rusty. If this reaction were not slow, we would not have very old iron structures in today's world.
- According to the type of particle involved.
- Reactions acid-base. Are transferred protons (H +). For example:
- Oxidation-reduction reactions. Are transferred electrons. In this type of reaction we must look at the oxidation number of the elements involved. If the oxidation number of an element increases, it is oxidized, if it decreases, it is reduced. For example: in this reaction iron is oxidized and cobalt is reduced.
- Reactions acid-base. Are transferred protons (H +). For example:
- According to the direction of the reaction.
- Reversible reactions. They go both ways, that is, the products can become the reactants again. For example:
- Irreversible reactions. They occur in only one sense, that is, the reactants are transformed into products and the opposite process cannot occur. For example:
- Reversible reactions. They go both ways, that is, the products can become the reactants again. For example:
Organic reactions. They involve organic compounds, which are those that are related to the basis of life. They depend on the type of organic compound for their classification, since each functional group has a range of specific reactions. For example, alkanes, alkenes, alkynes, alcohols, ketones, aldehydes, ethers, esters, nitriles, etc.
Some examples of reactions of organic compounds are:
- Halogenation of alkanes. A hydrogen of the alkane is replaced by the corresponding halogen.
- Combustion of alkanes. Alkanes react with oxygen to give carbon dioxide and water. This type of reaction releases a large amount of energy.
- Halogenation of alkenes. Two of the hydrogens present on the carbons that form the double bond are replaced.
- Hydrogenation of alkenes. Two hydrogens are added to the double bond, thus producing the corresponding alkane. This reaction occurs in the presence of catalysts such as platinum, palladium, or nickel.
Importance of chemical reactions
Both photosynthesis and respiration are examples of chemical reactions.Chemical reactions are fundamental to the existence and understanding of the world as we know it. The changes that matter undergoes under natural or man-made conditions (and that often generate valuable materials) are just one example. The greatest evidence of the importance of chemical reactions is life itself, in all its expressions.
The existence of living beings of all kinds is only possible thanks to the reaction capacity of matter, which allowed the first cellular forms of life to exchange energy with their environment through metabolic routes, that is, through sequences of chemical reactions that yielded more useful energy than consumed.
For example, in our daily life the breathing It is composed of multiple chemical reactions, which are also present in the photosynthesis of the plants.
Speed of a chemical reaction
Chemical reactions require a stipulated time to occur, which varies depending on the nature of the reactants and the environment in which the reaction occurs.
Factors that affect the rate of chemical reactions are generally:
- Temperature rise High temperatures tend to increase the speed of chemical reactions.
- Increased pressure. Increasing the pressure usually increases the speed of chemical reactions. This generally occurs when substances that are sensitive to pressure changes, such as gases, react. In the case of liquids and solids, pressure changes do not cause significant changes in the rate of their reactions.
- Aggregation state in which the reagents are. Solids tend to react more slowly than liquids or gases, although the speed will also depend on the reactivity of each substance.
- Use of catalysts (substances that are used to increase the speed of chemical reactions). These substances do not take part in the reactions, they only control the rate at which they occur. There are also substances called inhibitors, which are used in the same way but cause the opposite effect, that is, they slow down reactions.
- Luminous energy (Light). Some chemical reactions are accelerated when light is shined on them.
- Reagent concentration. Most chemical reactions happen faster if they have a high concentration of their reagents.

