- What are physical phenomena?
- Characteristics of physical phenomena
- Types of physical phenomena
- Examples of physical phenomena
- Physical phenomena and chemical phenomena
We explain what physical phenomena are, their characteristics, what types exist and various examples. Also, chemical phenomena.

What are physical phenomena?
Physical phenomena or physical changes are called changes in the state of the matter that are produced without altering its chemical composition, since they do not involve any type of chemical reactions. In the latter, they are distinguished, precisely, from chemical phenomena. They are mostly reversible.
Physical phenomena involve the set of forces that ordinarily affect the matter, as well as his change of State of aggregation: liquid, solid, gaseous or plasma. They may also have to do with mixture of substances, as long as they are heterogeneous mixtures, in which solvent and solute they do not present any type of permanent molecular bond.
Characteristics of physical phenomena
Physical phenomena, in principle, are observable with the naked eye, since the state of matter tends to change macroscopically. This is even more true for reversible physical changes.
However, in this type of phenomena the amount of matter is not altered, that is, the change does not imply a deep transformation of it, nor the creation or destruction of it, but simply the transition from one state to another, or from one structure to another.
Types of physical phenomena
Physical phenomena can be different, depending on their origin, usually in some of the physical forces of the universe. In this way, we can talk about:
- Movement. It occurs when a body changes its position of rest and moves from one point to another, or when it alters its trajectory and acquires a new one. All this as the effect of some kind of force on him, be it the gravity, the impact of some other body, etc. It is what happens when things fall to the ground, for example.
- Heat. It has to do with the level of Energy present in a body, that is, the speed and intensity with which its particles they agitate. Objects with higher internal energy will present temperatures higher, and those with lower energy, lower temperature. By adding heat to a body, it is possible to induce a change of state of aggregation, as when we boil Water and we turn it into gas, or when we freeze water and make it solid.
- Light. Electromagnetic radiation from energy sources such as Sun, affects the matter generating various phenomena. For example, him color of things is the result of light hitting objects and reflecting a single color among all those that make up the spectrum.
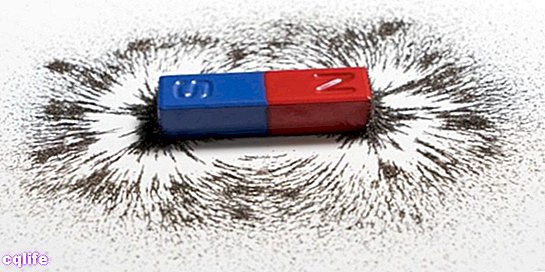
- Magnetism. Some metals (especially those linked to iron) have the ability to attract or repel other metals, due to their electronic configuration. These types of reactions do not alter the repelled or attracted metal, they simply reorganize the most superficial particles of its atoms.
- Electricity. Electricity and magnetism are closely related, since they come from the properties of electrons in the atoms of matter. But electricity, unlike magnetism, can be transmitted through certain materials known as drivers. Electricity is nothing more than the difference in electric potential between one point and another of matter, which generates a displacement electronic capable of being converted into other forms of energy: heat, light, movement, etc. A simple example of this is lightning: violent compensations of electric potential between the atmosphere and the I usually.
- Sound. The rhythmic vibration of certain bodies is capable of generating waves sounds that are transmitted in the air or water, thus generating sounds perceptible by the human or animal ear. The properties of sound depend on the vibrating matter and the medium of propagation of the waves. This is what happens when church bells are tolled.
Examples of physical phenomena
Some simple examples of physical phenomena are the changes of water states. In its natural state and at ordinary atmospheric pressure, water is liquid and transparent, just as it is when we drink it. If we add heat, heating it in a container, once it reaches 100 ° C the water will evaporate, converted into a gas (steam).
If, on the contrary, we remove heat by putting it in a freezer, once it reaches 0 ° C the water will begin to crystallize and eventually become solid (ice). All of these processes are reversible through the reverse mechanism: adding or removing heat.
Physical phenomena and chemical phenomena

As we said initially, the difference between physical phenomena and chemical phenomena has to do with the type of change caused in matter. In the first case, it is a change of structure, state, in which the substance remains chemically the same. For example, frozen water is still made up of hydrogen and oxygen.
Instead, chemical phenomena reorganize the molecular nature of matter, building and destroying atomic bonds and creating new substances. This is due to the fact that a chemical reaction occurs, generally irreversible, in which substances totally different from the initial ones are obtained.
For example, metals that react with oxygen are they oxidize, losing some of its properties, without being able to recover the oxygen, nor the transformed metal.
